Purines Purines A series of heterocyclic compounds that are variously substituted in nature and are known also as purine bases. They include adenine and guanine, constituents of nucleic acids, as well as many alkaloids such as caffeine and theophylline. Uric acid is the metabolic end product of purine metabolism. Nucleic Acids and pyrimidines Pyrimidines A family of 6-membered heterocyclic compounds occurring in nature in a wide variety of forms. They include several nucleic acid constituents (cytosine; thymine; and uracil) and form the basic structure of the barbiturates. Nucleic Acids are heterocyclic aromatic compounds, which, along with sugar and phosphate Phosphate Inorganic salts of phosphoric acid. Electrolytes groups, form the important components of nucleotides Nucleotides The monomeric units from which DNA or RNA polymers are constructed. They consist of a purine or pyrimidine base, a pentose sugar, and a phosphate group. Nucleic Acids. Purines Purines A series of heterocyclic compounds that are variously substituted in nature and are known also as purine bases. They include adenine and guanine, constituents of nucleic acids, as well as many alkaloids such as caffeine and theophylline. Uric acid is the metabolic end product of purine metabolism. Nucleic Acids include adenine Adenine A purine base and a fundamental unit of adenine nucleotides. Nucleic Acids and guanine Guanine Nucleic Acids, while pyrimidines Pyrimidines A family of 6-membered heterocyclic compounds occurring in nature in a wide variety of forms. They include several nucleic acid constituents (cytosine; thymine; and uracil) and form the basic structure of the barbiturates. Nucleic Acids include thymine Thymine One of four constituent bases of DNA. Nucleic Acids (in DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure), uracil Uracil One of four nucleotide bases in the nucleic acid RNA. Nucleic Acids (in RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure), and cytosine Cytosine A pyrimidine base that is a fundamental unit of nucleic acids. Nucleic Acids. Purine nucleotide synthesis Synthesis Polymerase Chain Reaction (PCR) follows a series of reactions using carbon donors, amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids (e.g., glutamine Glutamine A non-essential amino acid present abundantly throughout the body and is involved in many metabolic processes. It is synthesized from glutamic acid and ammonia. It is the principal carrier of nitrogen in the body and is an important energy source for many cells. Synthesis of Nonessential Amino Acids, aspartate Aspartate One of the non-essential amino acids commonly occurring in the l-form. It is found in animals and plants, especially in sugar cane and sugar beets. It may be a neurotransmitter. Synthesis of Nonessential Amino Acids), and bicarbonate Bicarbonate Inorganic salts that contain the -HCO3 radical. They are an important factor in determining the ph of the blood and the concentration of bicarbonate ions is regulated by the kidney. Levels in the blood are an index of the alkali reserve or buffering capacity. Electrolytes. The de novo pathway generates inosine monophosphate (IMP), which is the precursor of adenosine Adenosine A nucleoside that is composed of adenine and d-ribose. Adenosine or adenosine derivatives play many important biological roles in addition to being components of DNA and RNA. Adenosine itself is a neurotransmitter. Class 5 Antiarrhythmic Drugs monophosphate (AMP) and guanosine monophosphate (GMP). Purine synthesis Synthesis Polymerase Chain Reaction (PCR) is regulated in the 1st 2 steps. Synthesis Synthesis Polymerase Chain Reaction (PCR) of pyrimidine nucleotides Nucleotides The monomeric units from which DNA or RNA polymers are constructed. They consist of a purine or pyrimidine base, a pentose sugar, and a phosphate group. Nucleic Acids also follows different reactions, producing uridine monophosphate (UMP), which is converted to uridine triphosphate (UTP) and cytidine triphosphate (CTP). For thymine Thymine One of four constituent bases of DNA. Nucleic Acids, a part of deoxyribonucleotides Deoxyribonucleotides A purine or pyrimidine base bonded to a deoxyribose containing a bond to a phosphate group. DNA Types and Structure, ribonucleoside reductase is required to reduce the ribose Ribose A pentose active in biological systems usually in its d-form. Nucleic Acids moiety. Degradation of nucleotides Nucleotides The monomeric units from which DNA or RNA polymers are constructed. They consist of a purine or pyrimidine base, a pentose sugar, and a phosphate group. Nucleic Acids result in xanthine then uric acid Uric acid An oxidation product, via xanthine oxidase, of oxypurines such as xanthine and hypoxanthine. It is the final oxidation product of purine catabolism in humans and primates, whereas in most other mammals urate oxidase further oxidizes it to allantoin. Nephrolithiasis production in purines Purines A series of heterocyclic compounds that are variously substituted in nature and are known also as purine bases. They include adenine and guanine, constituents of nucleic acids, as well as many alkaloids such as caffeine and theophylline. Uric acid is the metabolic end product of purine metabolism. Nucleic Acids, while pyrimidines Pyrimidines A family of 6-membered heterocyclic compounds occurring in nature in a wide variety of forms. They include several nucleic acid constituents (cytosine; thymine; and uracil) and form the basic structure of the barbiturates. Nucleic Acids produce the amino acids Amino acids Organic compounds that generally contain an amino (-NH2) and a carboxyl (-COOH) group. Twenty alpha-amino acids are the subunits which are polymerized to form proteins. Basics of Amino Acids, β-alanine, and β-aminobutyrate.
Last updated: Sep 5, 2023
Nitrogenous base:
Nucleosides Nucleosides Purine or pyrimidine bases attached to a ribose or deoxyribose. Nucleic Acids: 2 components:
A beta-N-glycosidic bond links the 1st carbon of the pentose sugar and N9 of a purine or N1 of a pyrimidine (e.g., adenosine Adenosine A nucleoside that is composed of adenine and d-ribose. Adenosine or adenosine derivatives play many important biological roles in addition to being components of DNA and RNA. Adenosine itself is a neurotransmitter. Class 5 Antiarrhythmic Drugs, guanosine, cytidine, thymidine, uridine, inosine).
Nucleotides Nucleotides The monomeric units from which DNA or RNA polymers are constructed. They consist of a purine or pyrimidine base, a pentose sugar, and a phosphate group. Nucleic Acids: 3 main components:
These molecules form the DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure backbone (e.g., adenosine Adenosine A nucleoside that is composed of adenine and d-ribose. Adenosine or adenosine derivatives play many important biological roles in addition to being components of DNA and RNA. Adenosine itself is a neurotransmitter. Class 5 Antiarrhythmic Drugs monophosphate, guanosine monophosphate, cytidine monophosphate)
> 1 phosphate Phosphate Inorganic salts of phosphoric acid. Electrolytes groups:
Esterification Esterification The process of converting an acid into an alkyl or aryl derivative. Most frequently the process consists of the reaction of an acid with an alcohol in the presence of a trace of mineral acid as catalyst or the reaction of an Acyl chloride with an alcohol. Esterification can also be accomplished by enzymatic processes. Lipid Metabolism of the phosphate Phosphate Inorganic salts of phosphoric acid. Electrolytes groups forms the corresponding nucleoside diphosphates and triphosphates (e.g., adenosine Adenosine A nucleoside that is composed of adenine and d-ribose. Adenosine or adenosine derivatives play many important biological roles in addition to being components of DNA and RNA. Adenosine itself is a neurotransmitter. Class 5 Antiarrhythmic Drugs triphosphate (ATP), adenosine Adenosine A nucleoside that is composed of adenine and d-ribose. Adenosine or adenosine derivatives play many important biological roles in addition to being components of DNA and RNA. Adenosine itself is a neurotransmitter. Class 5 Antiarrhythmic Drugs diphosphate (ADP)).
Nucleic acid:
Polymer of nucleotides Nucleotides The monomeric units from which DNA or RNA polymers are constructed. They consist of a purine or pyrimidine base, a pentose sugar, and a phosphate group. Nucleic Acids (e.g., ribonucleic acid Ribonucleic acid A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure ( RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure)).
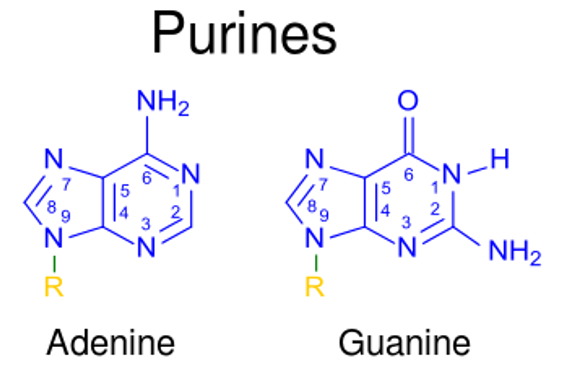
Structure of purines adenine and guanine
Image: “123” by Kevin Ahern. License: Public Domain, cropped by Lecturio.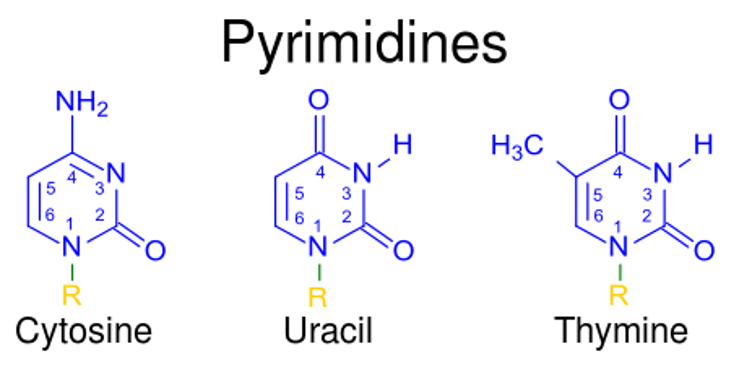
Pyrimidine nucleotides and their structures
Image: “123” by Kevin Ahern. License: Public Domain, cropped by Lecturio.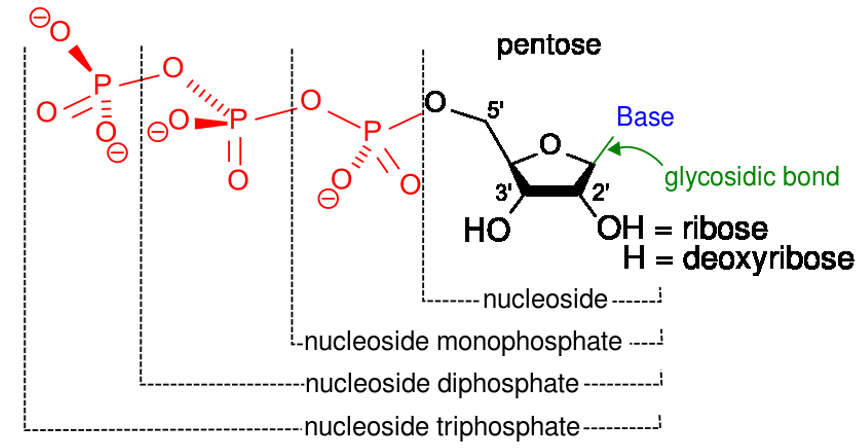
Components that build a nucleotide
Image: “123” by Kevin Ahern. License: Public Domain, cropped by Lecturio.The main functions of nucleotides Nucleotides The monomeric units from which DNA or RNA polymers are constructed. They consist of a purine or pyrimidine base, a pentose sugar, and a phosphate group. Nucleic Acids:
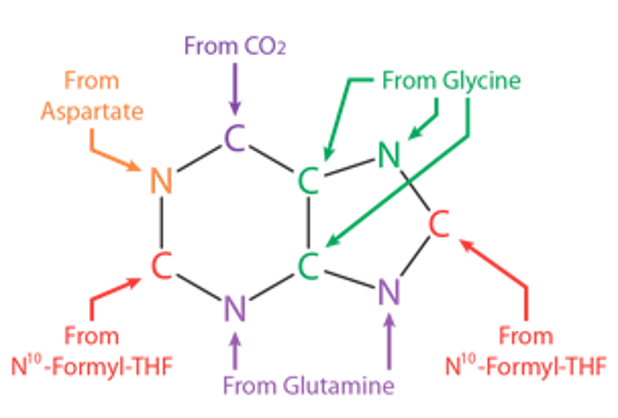
Atom sources for purine synthesis
THF: tetrahydrofolate
Synthesis Synthesis Polymerase Chain Reaction (PCR) of PRPP
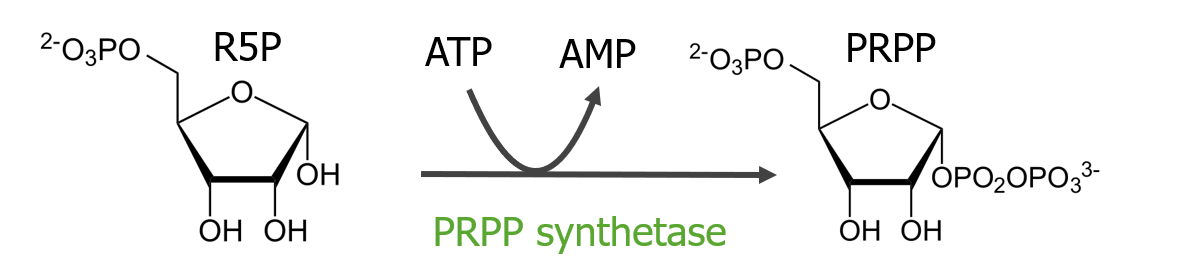
Synthesis of phosphoribosyl pyrophosphate (PRPP):
Ribose-5-phosphate (R5P) is converted to PRPP. The phosphates come from ATP and then produce AMP. The enzyme for the conversion is PRPP synthetase.
Formation of 5-phosphoribosylamine (PRA)
5-Phosphoribosylamine conversion to glycinamide ribonucleotide (GAR)
Formylation of GAR to formylglycinamide ribonucleotide (FGAR)
Conversion of FGAR to formylglycinamidine ribonucleotide (FGAM)
Formation of the purine imidazole ring Imidazole Ring Nitroimidazoles
Carboxylation of AIR
Formation of 5-aminoimidazole-4-(N-succinylcarboxamide) ribonucleotide (SAICAR)
Elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy of fumarate Fumarate Citric Acid Cycle
Formylation to form 5-formaminoimidazole-4-carboxamide ribonucleotide (FAICAR)
Cyclization to form IMP
| Step | Reaction | Added atom | Enzyme | Product |
|---|---|---|---|---|
| 1 | Ribose-5-phosphate Ribose-5-phosphate Pentose Phosphate Pathway → PRPP | Phosphates (from ATP) | PRPP synthetase | PRPP |
| 2 | PRPP + glutamine Glutamine A non-essential amino acid present abundantly throughout the body and is involved in many metabolic processes. It is synthesized from glutamic acid and ammonia. It is the principal carrier of nitrogen in the body and is an important energy source for many cells. Synthesis of Nonessential Amino Acids → 5-phosphoribosylamine | N9 (from glutamine Glutamine A non-essential amino acid present abundantly throughout the body and is involved in many metabolic processes. It is synthesized from glutamic acid and ammonia. It is the principal carrier of nitrogen in the body and is an important energy source for many cells. Synthesis of Nonessential Amino Acids) | Amidophosphoribosyltransferase | PRA |
| 3 | PRA conversion to GAR | C4, C5, N7 (from glycine Glycine A non-essential amino acid. It is found primarily in gelatin and silk fibroin and used therapeutically as a nutrient. It is also a fast inhibitory neurotransmitter. Synthesis of Nonessential Amino Acids) | GAR synthetase | GAR |
| 4 | Formylation of GAR to FGAR | C8 (from formyl THF) | GAR transformylase | FGAR |
| 5 | Conversion of FGAR to FGAM | N3 (from glutamine Glutamine A non-essential amino acid present abundantly throughout the body and is involved in many metabolic processes. It is synthesized from glutamic acid and ammonia. It is the principal carrier of nitrogen in the body and is an important energy source for many cells. Synthesis of Nonessential Amino Acids) | FGAM synthetase | FGAM |
| 6 | Ring closure, forming AIR | AIR synthetase | AIR | |
| 7 | Carboxylation of AIR | C6 (from bicarbonate Bicarbonate Inorganic salts that contain the -HCO3 radical. They are an important factor in determining the ph of the blood and the concentration of bicarbonate ions is regulated by the kidney. Levels in the blood are an index of the alkali reserve or buffering capacity. Electrolytes) | AIR carboxylase | AICAR |
| 8 | Formation of SAICAR | N1 (from aspartate Aspartate One of the non-essential amino acids commonly occurring in the l-form. It is found in animals and plants, especially in sugar cane and sugar beets. It may be a neurotransmitter. Synthesis of Nonessential Amino Acids) | SAICAR synthetase | SAICAR |
| 9 | Fumarate Fumarate Citric Acid Cycle removed AICAR formed | Adenylosuccinate lyase | AICAR | |
| 10 | FAICAR formed | C2 (from formyl-THF) | AICAR transformylase | FAICAR |
| 11 | IMP formed | IMP cyclohydrolase | IMP |
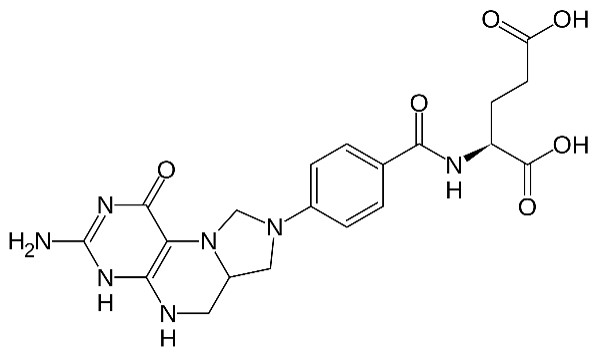
Structure of folate
Image by Lecturio.Inosine monophosphate is converted to adenine Adenine A purine base and a fundamental unit of adenine nucleotides. Nucleic Acids and guanine Guanine Nucleic Acids as AMP and GMP. Formed from GMP, guanosine triphosphate (GTP) provides the energy to convert IMP to AMP.
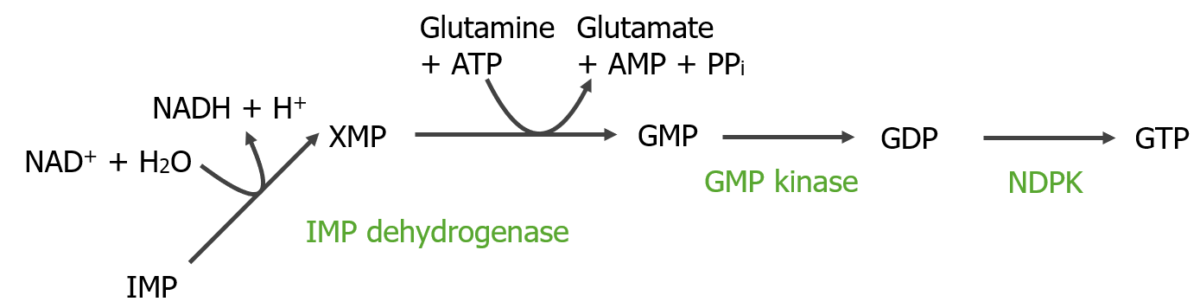
Conversion of IMP to GMP and then to GTP:
NAD+: nicotinamide adenine dinucleotide (oxidized)
NADH: nicotinamide adenine dinucleotide (reduced)
NDPK: nucleoside diphosphate kinase
PPi: pyrophosphate
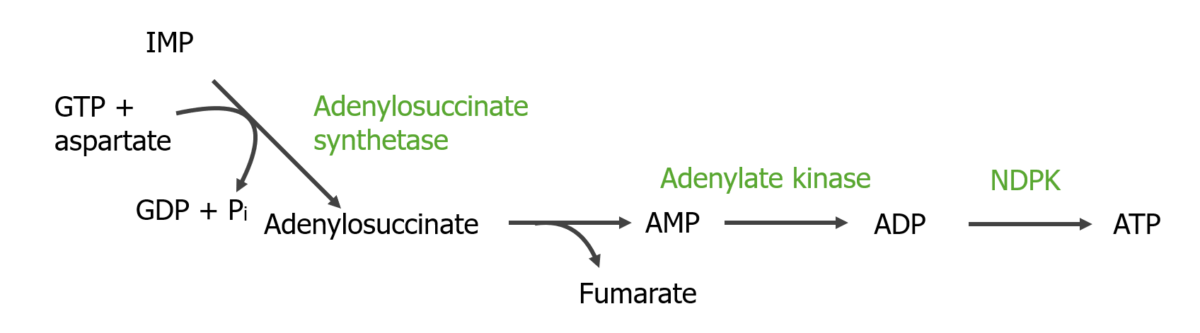
Conversion of IMP to AMP and then to ATP:
NDPK: nucleoside diphosphate kinase
Pi: inorganic phosphate
Synthesis Synthesis Polymerase Chain Reaction (PCR) of IMP, ATP and GTP is regulated to control the amount of purine nucleotides Nucleotides The monomeric units from which DNA or RNA polymers are constructed. They consist of a purine or pyrimidine base, a pentose sugar, and a phosphate group. Nucleic Acids produced.
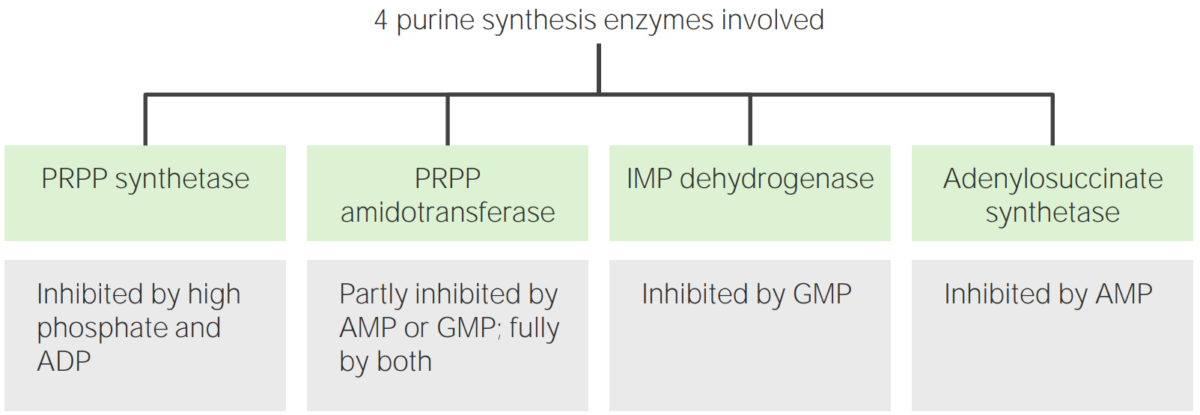
Regulators of purine metabolism
Image by Lecturio.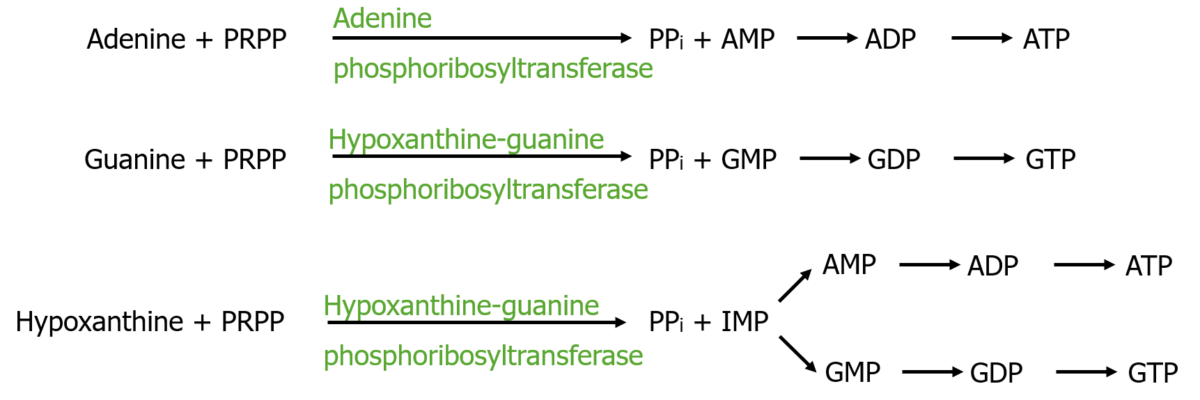
The salvage pathway that recycles nucleotides for utilization
Image by Lecturio.Nucleic acid ( RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure/ DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure) is broken down by nucleases Nucleases Pancreatic Parameters to nucleotides Nucleotides The monomeric units from which DNA or RNA polymers are constructed. They consist of a purine or pyrimidine base, a pentose sugar, and a phosphate group. Nucleic Acids. To degrade purine nucleotides Nucleotides The monomeric units from which DNA or RNA polymers are constructed. They consist of a purine or pyrimidine base, a pentose sugar, and a phosphate group. Nucleic Acids, the phosphate Phosphate Inorganic salts of phosphoric acid. Electrolytes and ribose Ribose A pentose active in biological systems usually in its d-form. Nucleic Acids are removed first, with further reactions leading to xanthine and then to uric acid Uric acid An oxidation product, via xanthine oxidase, of oxypurines such as xanthine and hypoxanthine. It is the final oxidation product of purine catabolism in humans and primates, whereas in most other mammals urate oxidase further oxidizes it to allantoin. Nephrolithiasis.
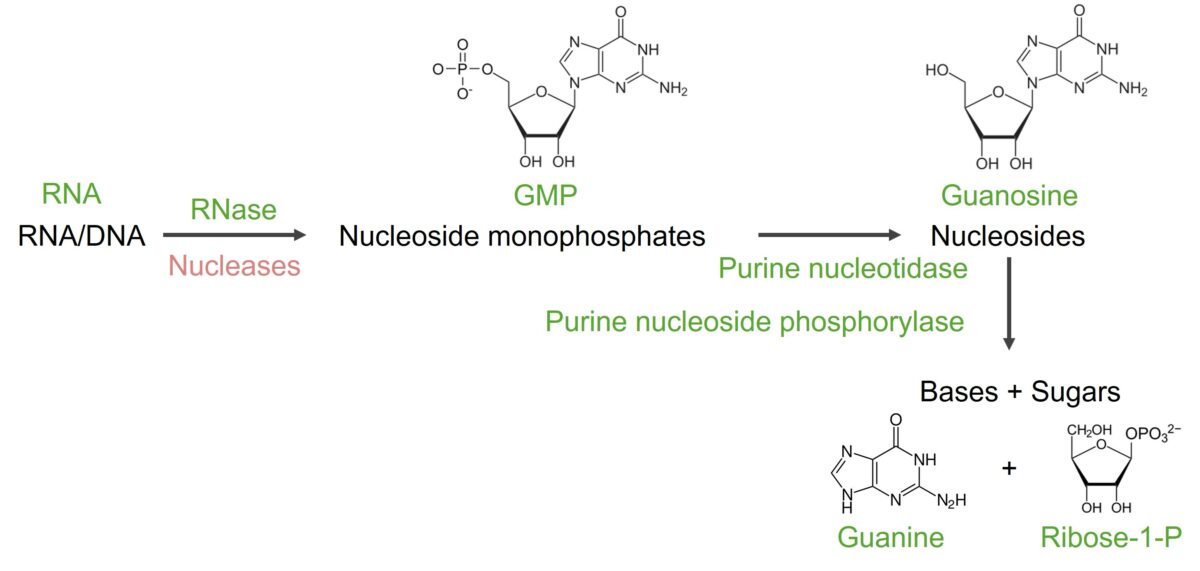
Degradation of guanine
Image by Lecturio.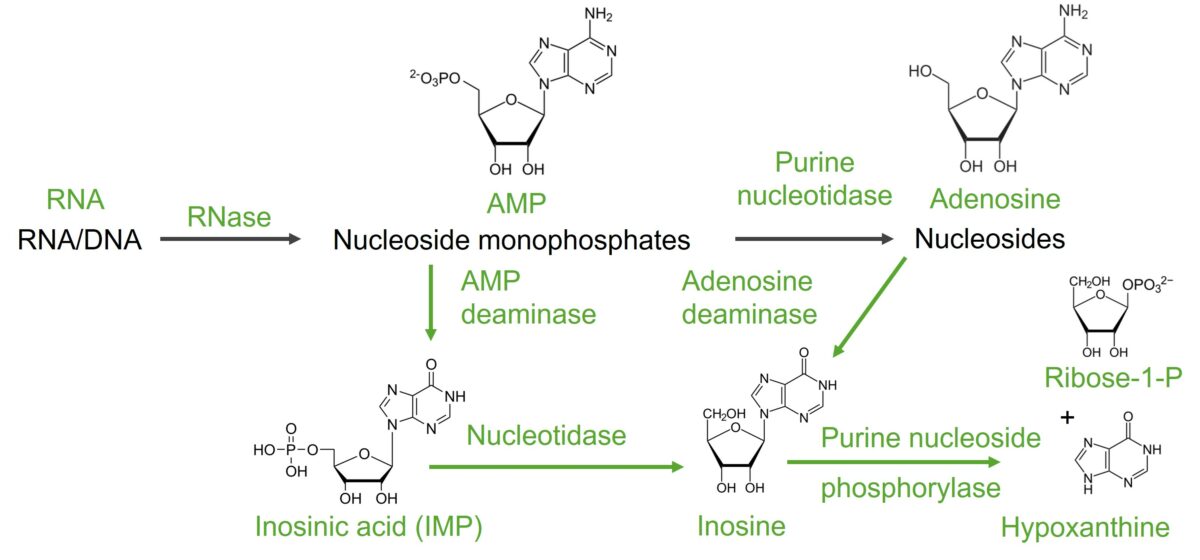
Degradation of adenine
Image by Lecturio.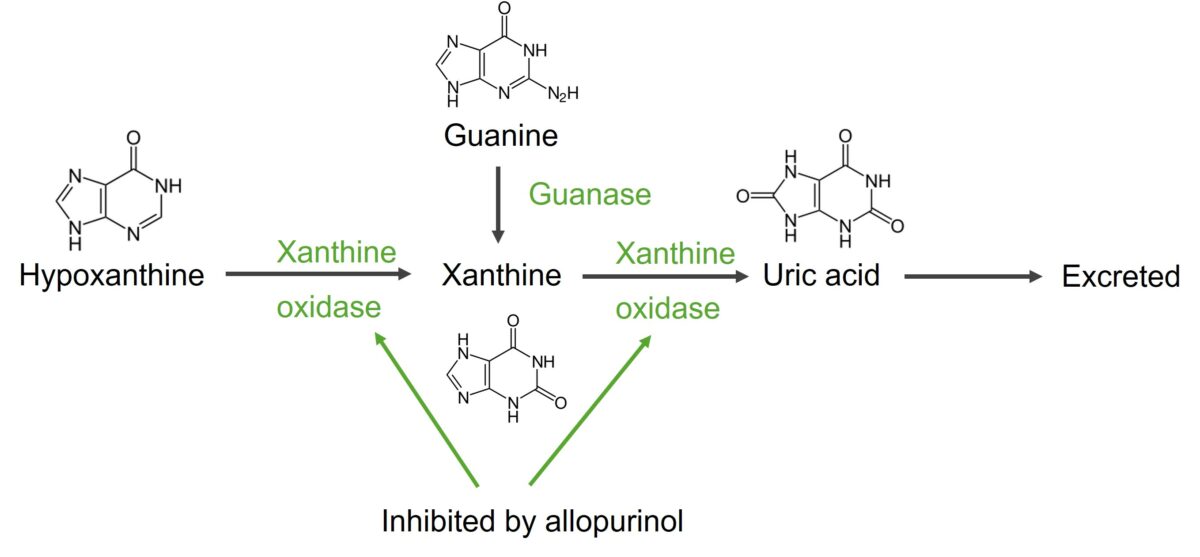
Degradation of guanine and hypoxanthine into uric acid
Image by Lecturio.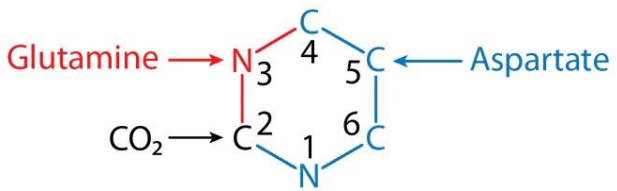
Sources of the carbon and nitrogen atoms in pyrimidine synthesis
Image by Lecturio.Synthesis Synthesis Polymerase Chain Reaction (PCR) of carbamoyl phosphate Phosphate Inorganic salts of phosphoric acid. Electrolytes
Synthesis Synthesis Polymerase Chain Reaction (PCR) of carbamoyl aspartate Aspartate One of the non-essential amino acids commonly occurring in the l-form. It is found in animals and plants, especially in sugar cane and sugar beets. It may be a neurotransmitter. Synthesis of Nonessential Amino Acids
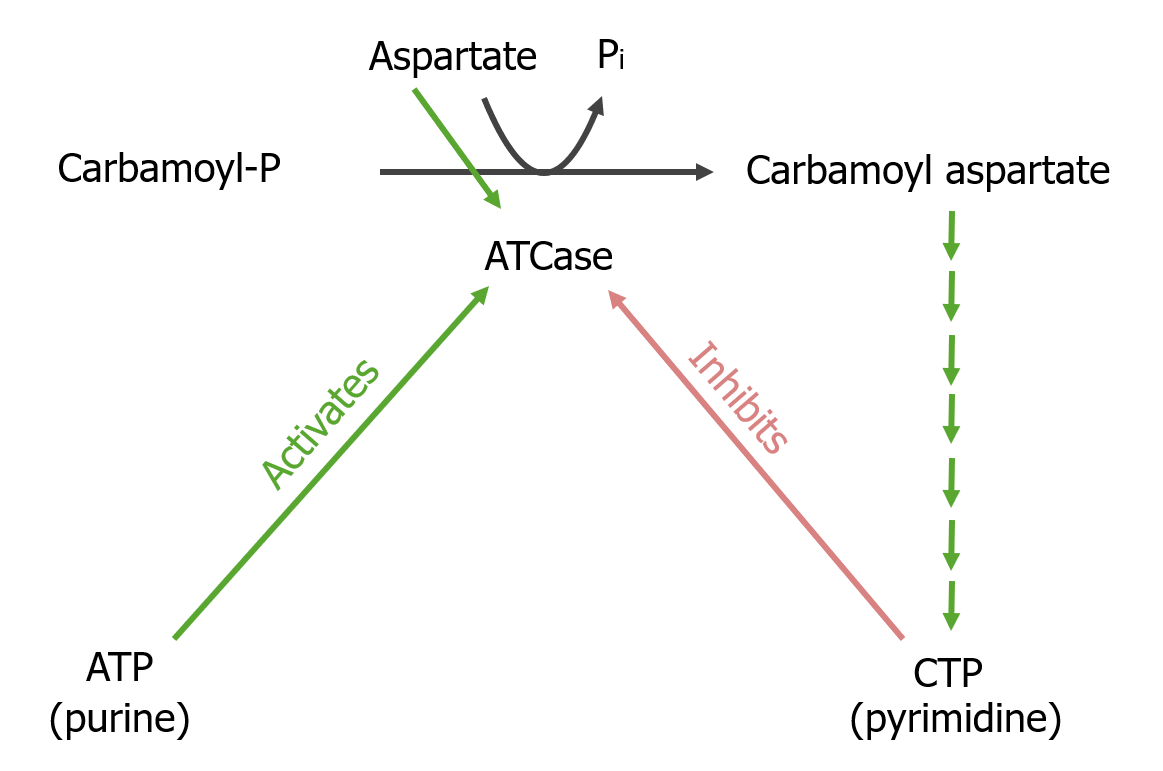
Rate-limiting step of pyrimidine synthesis:
Reaction converts carbamoyl phosphate to carbamoyl aspartate, catalyzed by aspartyl transcarbamoylase (ATCase). Subsequent reactions eventually lead to the end product, cytidine triphosphate (CTP). The ATCase is activated by ATP and inhibited by CTP.
Formation of the pyrimidine ring
Oxidation of dihydroorotate
Formation of orotidine-5-monophosphate (OMP)
Decarboxylation Decarboxylation The removal of a carboxyl group, usually in the form of carbon dioxide, from a chemical compound. Catabolism of Amino Acids to form uridine monophosphate (UMP)
Note: The last 2 enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body’s constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes in this pathway, OPRT and OMP decarboxylase, are located on the same polypeptide, UMP synthase. UMP synthase catalyzes the conversion of orotic acid to UMP.
| Step | Enzyme | Product |
|---|---|---|
| 1 | Carbamoyl phosphate Phosphate Inorganic salts of phosphoric acid. Electrolytes synthetase II | Carbamoyl phosphate Phosphate Inorganic salts of phosphoric acid. Electrolytes |
| 2 | Aspartyl transcarbamoylase* | Carbamoyl aspartate Aspartate One of the non-essential amino acids commonly occurring in the l-form. It is found in animals and plants, especially in sugar cane and sugar beets. It may be a neurotransmitter. Synthesis of Nonessential Amino Acids |
| 3 | Dihydroorotase | Dihydroorotic acid |
| 4 | Dihydroorotate dehydrogenase | Orotic acid |
| 5 | Orotate phosphoribosyltransferase | OMP |
| 6 | OMP decarboxylase | Uridine monophosphate |
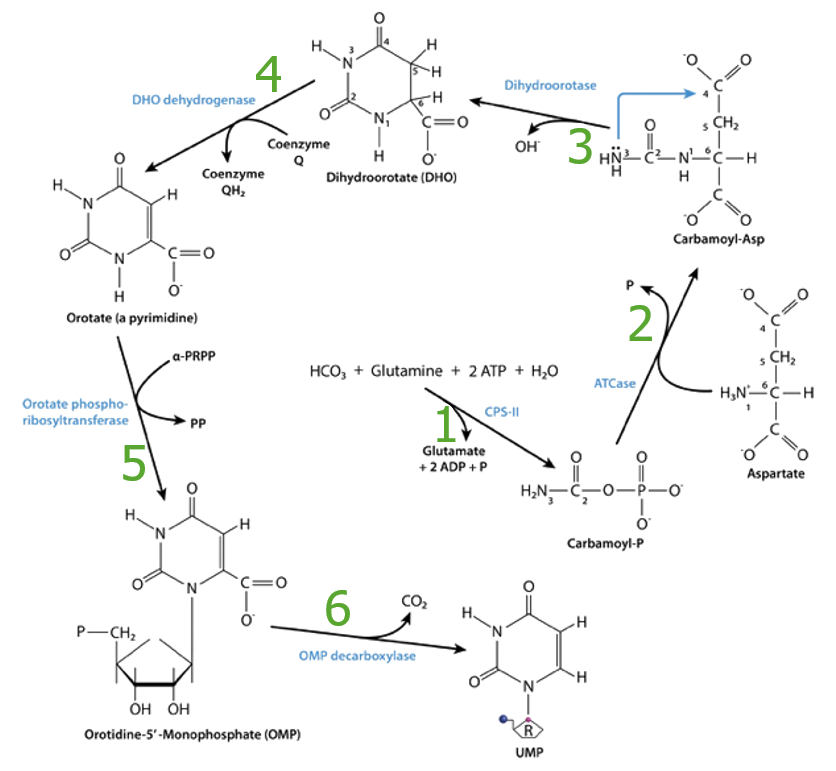
Summary of pyrimidine synthesis, enzymes:
1. CPS II: carbamoyl phosphate synthetase II
2. ATCase: aspartyl transcarbamoylase
3. Dihydroorotase
4. Dihydroorotate (DHO) dehydrogenase
5. Orotate phosphoribosyltransferase
6. Orotidine-5-monophosphate (OMP) decarboxylase
UTP and CTP are used in the synthesis Synthesis Polymerase Chain Reaction (PCR) of RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure.
UTP:
CTP:
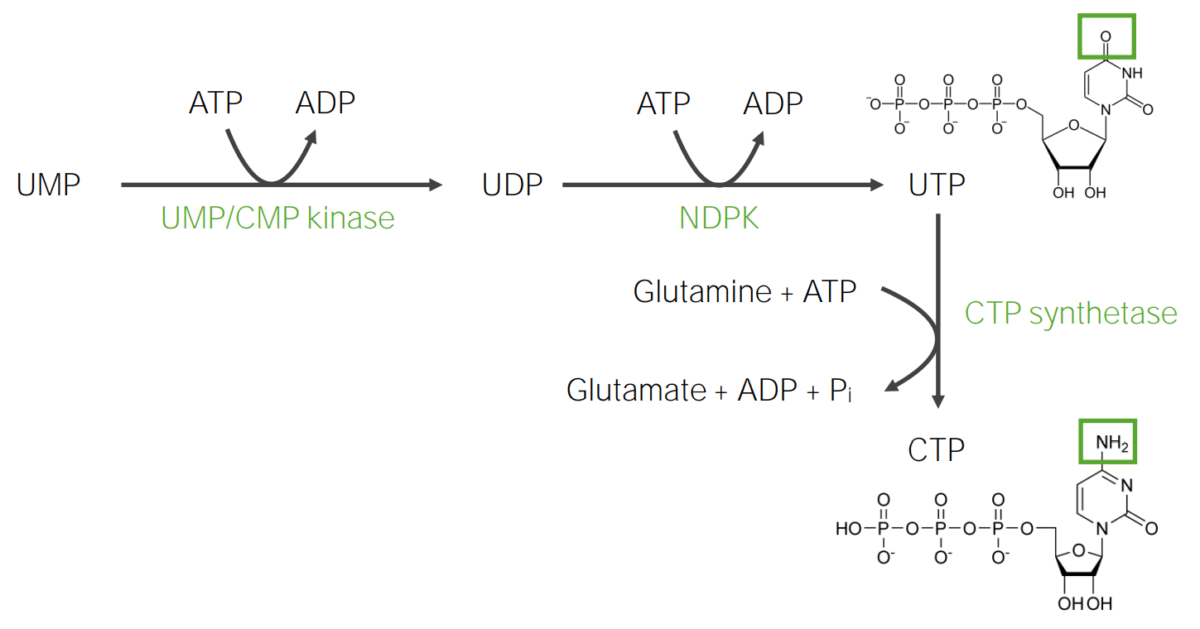
Synthesis of UTP and CTP (triphosphates)
Image by Lecturio.DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure is different from RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure, as DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure has deoxyribose Deoxyribose Nucleic Acids, instead of ribose Ribose A pentose active in biological systems usually in its d-form. Nucleic Acids, and thymine Thymine One of four constituent bases of DNA. Nucleic Acids (5-methyluracil), instead of uracil Uracil One of four nucleotide bases in the nucleic acid RNA. Nucleic Acids.
Deoxyribonucleotides Deoxyribonucleotides A purine or pyrimidine base bonded to a deoxyribose containing a bond to a phosphate group. DNA Types and Structure are generated from their corresponding ribonucleotides.
Thymine Thymine One of four constituent bases of DNA. Nucleic Acids is a pyrimidine present in DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure; thus, the ribose Ribose A pentose active in biological systems usually in its d-form. Nucleic Acids moiety of the corresponding nucleotide requires reduction.
Clinical correlation Correlation Determination of whether or not two variables are correlated. This means to study whether an increase or decrease in one variable corresponds to an increase or decrease in the other variable. Causality, Validity, and Reliability: 5-fluorouracil 5-Fluorouracil A pyrimidine analog that is an antineoplastic antimetabolite. It interferes with DNA synthesis by blocking the thymidylate synthetase conversion of deoxyuridylic acid to thymidylic acid. Antimetabolite Chemotherapy: antimetabolite agent (used in cancers) that inhibits thymidylate synthase and decreases DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure synthesis Synthesis Polymerase Chain Reaction (PCR)
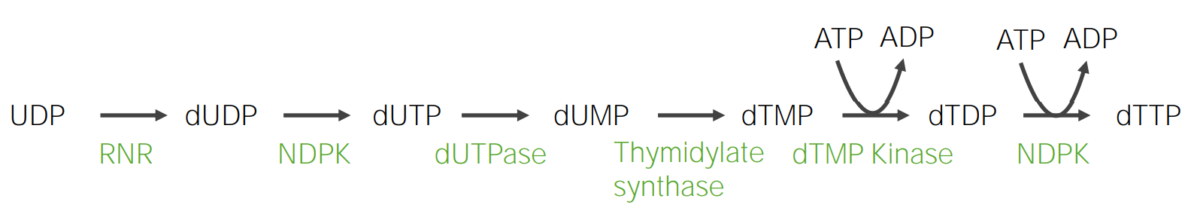
Formation of thymine in the form of deoxythymidine triphosphate (dTTP)
dTDP: deoxythymidine diphosphate
dTMP: deoxythymidine monophosphate
dTTP: deoxythymidine triphosphate
dUDP: deoxyuridine diphosphate
dUMP: deoxyuridine monophosphate
dUTPase: deoxyuridine triphosphatase
NDPK: nucleoside diphosphate kinase
RNR: ribonucleotide reductase
UDP: uridine diphosphate
Animal cells break down pyrimidine nucleotides Nucleotides The monomeric units from which DNA or RNA polymers are constructed. They consist of a purine or pyrimidine base, a pentose sugar, and a phosphate group. Nucleic Acids to the nitrogenous bases Nitrogenous bases Nucleic Acids, with the resultant uracil Uracil One of four nucleotide bases in the nucleic acid RNA. Nucleic Acids and thymine Thymine One of four constituent bases of DNA. Nucleic Acids degraded (via reduction) in the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy.
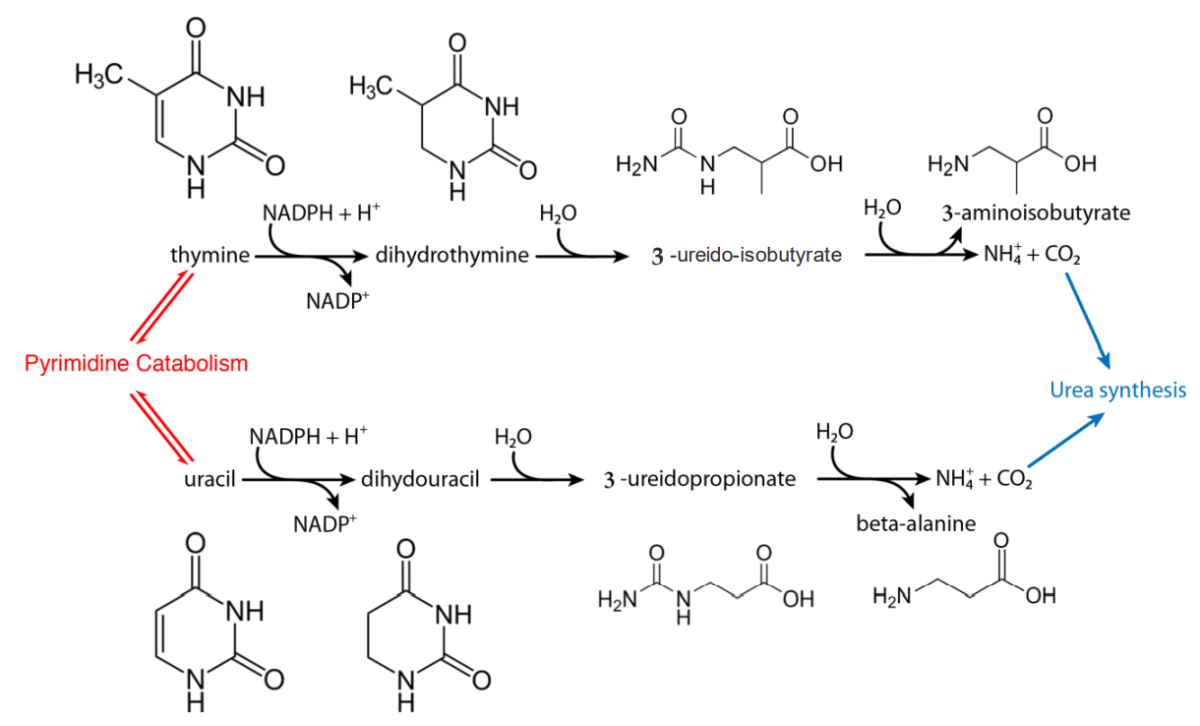
Degradation of uracil and thymine
NADPH: nicotinamide adenine dinucleotide phosphate
| Disorder | Defective enzyme | Nature of defect | Manifestations |
|---|---|---|---|
| Hyperuricemia Hyperuricemia Excessive uric acid or urate in blood as defined by its solubility in plasma at 37 degrees c; greater than 0. 42 mmol per liter (7. 0 mg/dl) in men or 0. 36 mmol per liter (6. 0 mg/dl) in women. Gout/ gout Gout Gout is a heterogeneous metabolic disease associated with elevated serum uric acid levels (> 6.8 mg/dL) and abnormal deposits of monosodium urate in tissues. The condition is often familial and is initially characterized by painful, recurring, and usually monoarticular acute arthritis, or “gout flare,” followed later by chronic deforming arthritis. Gout |
|
↑ Uric acid Uric acid An oxidation product, via xanthine oxidase, of oxypurines such as xanthine and hypoxanthine. It is the final oxidation product of purine catabolism in humans and primates, whereas in most other mammals urate oxidase further oxidizes it to allantoin. Nephrolithiasis | Inflamed and painful joints |
| Lesch-Nyhan syndrome Lesch-Nyhan syndrome An inherited disorder transmitted as a sex-linked trait and caused by a deficiency of an enzyme of purine metabolism; hypoxanthine phosphoribosyltransferase. Elevation of uric acid in the serum leads to the development of renal calculi and gouty arthritis. Purine Salvage Deficiencies | ↓ HGPRT | Lack of enzyme → defective purine salvage pathway |
|
| SCID SCID Severe combined immunodeficiency (SCID), also called “bubble boy disease,” is a rare genetic disorder in which the development of functional B and T cells is disturbed due to several genetic mutations that result in reduced or absent immune function. Severe Combined Immunodeficiency (SCID) | ↓ ADA | Lack of enzyme → ↓ immune cells |
|
| Renal lithiasis | ↓ APRT | Autosomal recessive Autosomal recessive Autosomal inheritance, both dominant and recessive, refers to the transmission of genes from the 22 autosomal chromosomes. Autosomal recessive diseases are only expressed when 2 copies of the recessive allele are inherited. Autosomal Recessive and Autosomal Dominant Inheritance mutation Mutation Genetic mutations are errors in DNA that can cause protein misfolding and dysfunction. There are various types of mutations, including chromosomal, point, frameshift, and expansion mutations. Types of Mutations → defective purine salvage pathway |
|
| Xanthinuria | ↓ Xanthine oxidase Oxidase Neisseria | Hypouricemia |
|
| Disorder | Defective enzyme | Manifestations |
|---|---|---|
| Orotic aciduria Orotic aciduria Orotic aciduria is an extremely rare genetic disorder that can result in crystalluria, megaloblastic anemia, developmental delay, and failure to thrive. The disorder is caused by an enzyme deficiency in the pyrimidine synthesis pathway resulting in the accumulation of orotic acid. Orotic Aciduria |
|
|
| Drug-induced orotic aciduria Orotic aciduria Orotic aciduria is an extremely rare genetic disorder that can result in crystalluria, megaloblastic anemia, developmental delay, and failure to thrive. The disorder is caused by an enzyme deficiency in the pyrimidine synthesis pathway resulting in the accumulation of orotic acid. Orotic Aciduria | OMP decarboxylase |
|